Summary
SeaStar Medical Receives FDA Approval to Begin Study with Selective Cytopheretic Device to Reduce Hyperinflammation in Adults with Acute Kidney Injury.
The Company plans to begin enrollment in this 200-patient randomized, controlled trial in March 2023.
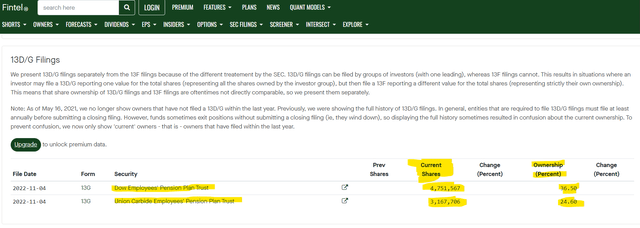
Dow Employees’ Pension Plan Trust &.Union Carbide Employees’ Pension Plan Trust hold close to 8 million shares equivalent to more than 60% ownership.
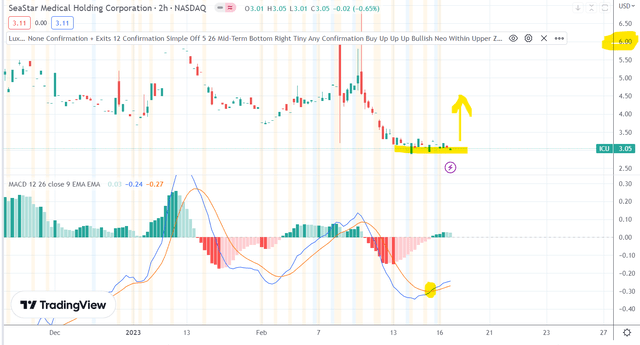
Chart suggests that $ICU is bottomed, great entry point. I expect it will rally 50% minimum.
SeaStar could be the potential takeover target from the big companies.
SeaStar Medical ($ICU), a medical device company developing proprietary solutions to reduce the consequences of hyperinflammation on vital organs, announces today that the U.S. Food and Drug Administration (FDA) approved the Company’s investigational device exemption (IDE) application to conduct a pivotal study evaluating the effectiveness of its Selective Cytopheretic Device (SCD) in reducing hyperinflammation in adults with acute kidney injury (AKI) requiring continuous kidney replacement therapy (CKRT).
The Company plans to begin enrollment in this 200-patient randomized, controlled trial in March 2023.
We currently expect to generate interim study results during the fourth quarter of 2023 and topline study results and submission of a Pre-market Approval (PMA) application in the second half of 2024. The study’s primary endpoint is a composite of 90-day mortality and dialysis dependency of patients treated with SCD in addition to CKRT standard of care, compared with the control group receiving CKRT standard of care
The Company’s innovative SCD is a patented cell-directed extracorporeal therapy that selectively targets the most highly activated pro-inflammatory neutrophils and monocytes to stop the cytokine storm that can cause organ failure and death. SCD therapy is currently delivered through continuous CKRT to target and neutralize pro-inflammatory neutrophils and monocytes, allowing the body to return to homeostasis. The SCD received FDA Breakthrough Device Designation in May 2022.
Approximately six million cases of adult AKI are diagnosed annually in the U.S, of which 200,000 require CKRT. The SCD has previously demonstrated success in critically ill adults with AKI requiring CKRT, a condition with a high mortality rate. In the Company’s SCD 005 pilot study evaluating the safety and feasibility of the SCD in COVID-19 patients with AKI and/or acute respiratory distress syndrome (ARDS), patients experienced reductions in activated neutrophils and monocytes, which led to reduction in proinflammatory cytokines and improved clinical outcomes. Based on the per-protocol minimum of four days of therapy, mortality of treated patients was significantly lower (41%) than the control population treated under standard of care (81%). All patients in the study received CKRT as the SCD delivery vehicle.
Dow Employees’ Pension Plan Trust &.Union Carbide Employees’ Pension Plan Trust hold close to 8 million shares equivalent to more than 60% ownership.